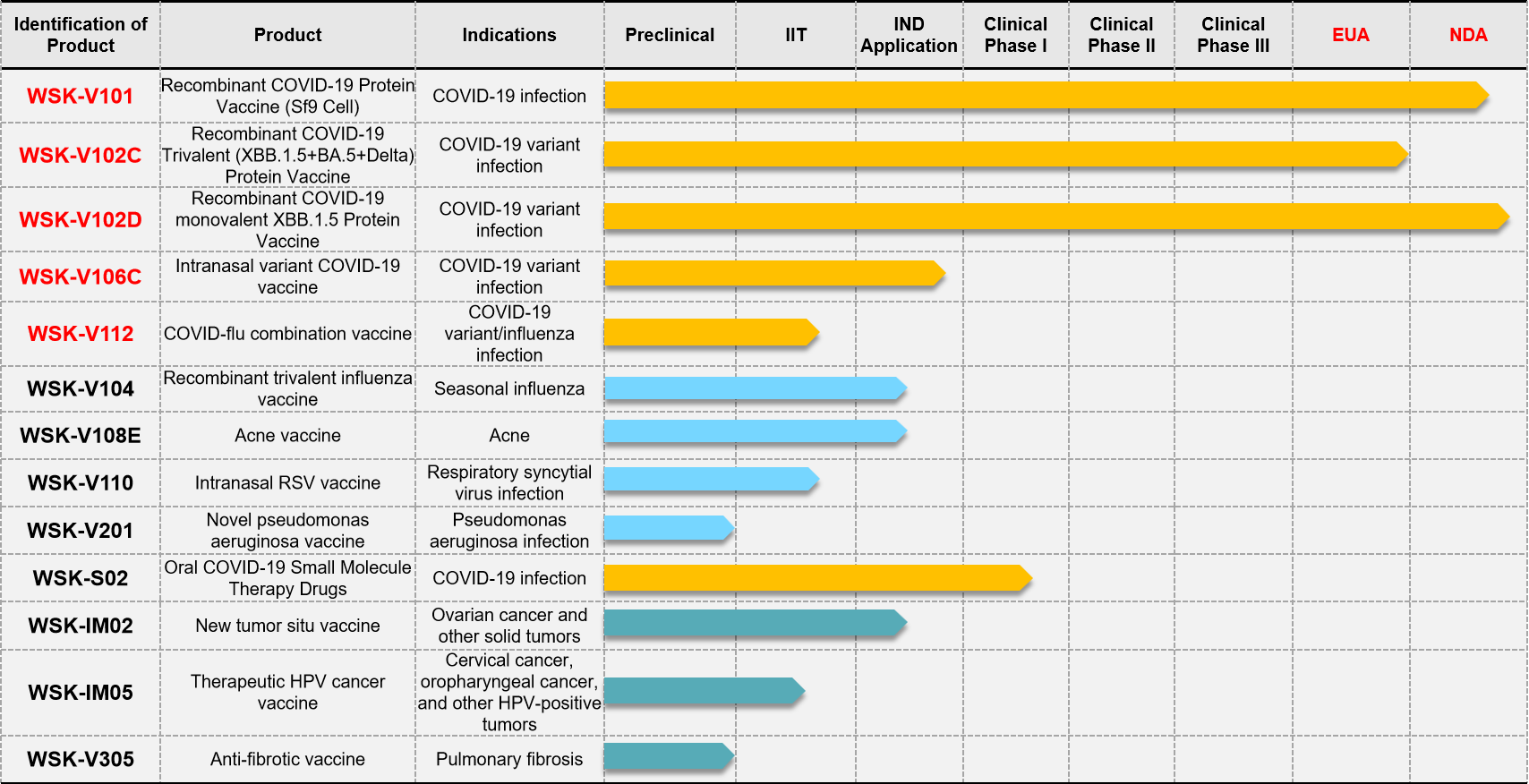
Introduction to the current key R&D pipeline:
1. Recombinant trivalent influenza vaccine (WSK-V104): It’s the first trivalent influenza vaccine in China prepared using insect cell-expressed recombinant proteins, and is in line with the latest WHO recommendations. It offers good safety and a broad target population (including those allergic to eggs). Among individuals aged 50 and above, this recombinant influenza vaccine demonstrates superior protective efficacy compared to egg culture-based inactivated influenza vaccines (with over 30% better protection). IND application is currently in progress.
2. Acne vaccine (WSK-V108E): It’s the first recombinant protein acne vaccine in China for the prevention and treatment of acne. There are currently no therapeutic acne vaccines available on the market domestically or internationally, indicating a huge market potential. IND application is currently in progress.
3. New tumor situ vaccine (WSK-IM02): It’s an in situ vaccine targeting multiple solid tumors, and is the first of its kind internationally to use an oxidized nucleic acid/liposome design, and has been granted patents in multiple countries including China, the United States, Japan, and Europe. It features a unique mechanism of action, good safety profile, and can inhibit the growth and metastasis of various tumors. It also shows enhanced effects when used in combination with immune checkpoint inhibitors and offers low cost. IND application is currently in progress.
4. Intranasal Respiratory Syncytial Virus vaccine (WSK-V110): It’s the first intranasal Respiratory Syncytial Virus (RSV) vaccine internationally. It features a novel structure and is free from intellectual property disputes. It has shown excellent protective effects in challenge experiments. An IND application is planned in the near future.
5. Recombinant COVID-flu combination vaccine (WSK-V112): There is currently no COVID-flu combination vaccine on the market internationally. It is the only company in China with a mature insect cell expression platform capable of producing both COVID-19 and influenza vaccines. The COVID-flu combination vaccine is expected to be the fastest product to apply for approval in China. An IND application is planned in the near future.
6. Therapeutic HPV cancer vaccine (WSK-IM05): It’s the first therapeutic HPV cancer vaccine internationally using a nasal spray formulation for the treatment of oropharyngeal cancer caused by HPV infection. It uses a unique sequence design with good tumor-suppression effects. An IIT clinical trial is currently underway, and an IND application is planned in the near future.
7. Anti-fibrotic vaccine (WSK-V304): There is currently no preventive or therapeutic anti-fibrosis vaccine available globally. This recombinant vaccine, with a novel design and structure, offers significant preventive and therapeutic effects against pulmonary fibrosis. An IIT clinical trial application is currently in progress.