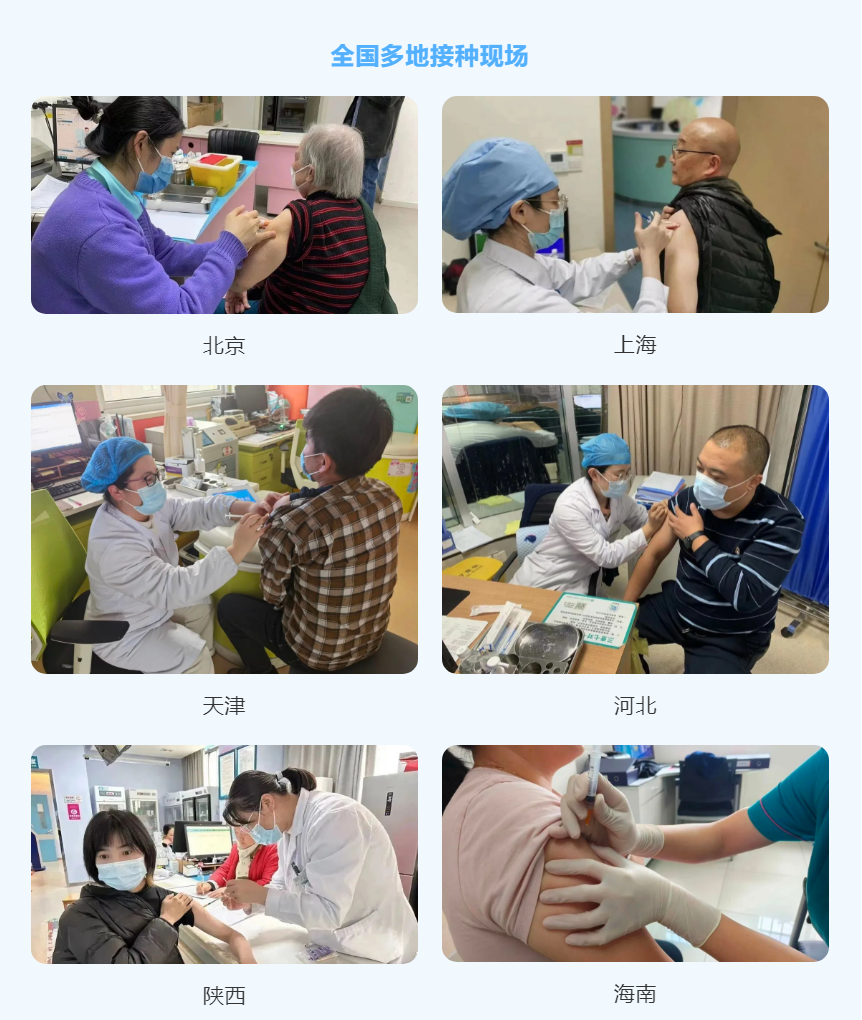
Coviccine® Trivalent XBB Vaccine Widely Administered! Further Fortifying the National Immune Wall
As the weather gets colder in the fall and winter, there may be a risk of simultaneous or concurrent outbreaks of respiratory infectious diseases such as COVID-19 and influenza, leading to a new peak of respiratory infections. It is imperative to take preventive measures in advance.
According to the report from the Chinese Center for Disease Control and Prevention, since 2023, the proportion of XBB variant strains and their sublineages in China has gradually increased, reaching 99% by the end of August. The XBB variant strains are a recombinant variant of Omicron, with stronger transmission and immune evasion capabilities than the early circulating Omicron variant, making it the dominant strain globally.
In late July 2023, the "Work Program for COVID-19 vaccine administration of Key Populations in the Near Future" (hereinafter referred to as the "vaccine administration program") was issued according to the Joint Prevention and Control Mechanism of the State Council for COVID-19. The vaccine administration program clearly states that during the prevalence of the XBB variant of COVID-19, especially during the autumn and winter of this year, administration with vaccines containing XBB variant antigens should be prioritized. Recombinant COVID-19 Trivalent Protein Vaccine (Sf9 Cell) - Coviccine® Trivalent Vaccine developed by WestVac BioPharma and Sichuan University West China Hospital has been officially launched in multiple regions across the country.
Coviccine® Trivalent Vaccine is now available in all areas of China.
Studies have shown that the XBB variant strains have a strong immune escape ability, with poor neutralizing capacity of serological antibodies in the Chinese population, thereby increasing the risk of breakthrough infections, especially in elderly individuals and other high-risk groups. The Vaccination Plan states that individuals aged 60 and above who have completed the primary immunization or have been infected with the COVID-19, as well as individuals aged 18 to 59 with severe underlying diseases, immunodeficiency, or high infection risk(including: relevant personnel in key industries such as civil affairs, health, education, housing and construction, transportation, public security, justice, state-owned assets, finance, market, culture and tourism, industry and information technology, commerce, technology, human resources and social security), can receive one dose of the vaccine containing XBB variant antigens 3-6 months after the most recent vaccination or 6 months after the most recent infection (whichever is more recent), according to XX, an expert of disease control and prevention.

According to the latest research published in Nature, it is estimated that the annual infection rate of COVID-19 in the future will be around 50%, while the influenza virus infection rate will be around 20%. A study in the UK has shown that compared to a single infection of influenza, the combination of influenza and COVID-19 infection can increase the severity and mortality rate in patients, about six times higher than in patients infected with neither of virus. Meanwhile, compared with those who were not re-infected, individuals who experience reinfection of COVID-19 face a 2.17 times higher risk of death and a 3.32 times higher risk of hospitalization, as well as an increased risk of post-infection complications in multiple systems such as the pulmonary, cardiovascular, blood, diabetes, and nervous systems.

In July of this year, the "Work Program for COVID-19 vaccine administration of Key Populations in the Near Future" (hereinafter referred to as the "Vaccination Program") was issued according to the Joint Prevention and Control Mechanism of the State Council for COVID-19. The Coviccine® Trivalent Vaccine has now been included for emergency use within a certain scope and is available for vaccination among prioritized populations in the near term. "Since the release of the Vaccination Plan, many residents have come to inquire about the possibility of receiving the latest COVID-19 vaccine," said Bai Lin, Head of the Immunization Department at the Fangcao Community Health Service Center in Chengdu High-tech Zone. "In fact, the 'China Influenza Vaccination Technical Guidelines 2022' also pointed out that it is safe and immunogenic for individuals aged 18 and above to receive both the influenza vaccine and the COVID-19 vaccine simultaneously. Therefore, we recommend that everyone receive both vaccines at the same time, which not only reduces the risk of severe illness following infection by the influenza virus or COVID-19 but also improves the efficiency of immunization and reduces the number of visits to vaccination clinics."

The Coviccine® Trivalent Vaccine, which has been launched for vaccination at the Fangcao Community Health Service Center, is the world's first broad-spectrum COVID-19 vaccine developed by the team led by Academician Wei Yuquan in conjunction with Sichuan University West China Hospital and WestVac BioPharma, effectively preventing diseases caused by multiple variants including the XBB and other variants of COVID-19. It provides excellent protection against current XBB variant strains of COVID-19 and citizens can feel confident in getting vaccinated," said Director Li Shuangqing from the General Medicine Department of West China Hospital, Sichuan University.

It is reported that the Coviccine® Trivalent Vaccine has been shipped and will gradually arrive at various national disease control centers and vaccination units. People who are eligible for vaccination can consult with the relevant disease control centers and vaccination units to make an appointment for vaccination. "We have been paying attention to the development of COVID-19 vaccines targeting the XBB variant domestically, and we are looking forward to the trivalent vaccine from Sichuan University West China Hospital / WestVac BioPharma. I heard that it is available for vaccination in Chengdu and I will promptly make an appointment for vaccination," said xx, a citizen of Chengdu.
Precise, potent, and improved safety
The Coviccine® Trivalent Vaccine is a high-purity and high-quality recombinant trivalent vaccine against COVID-19 variants produced by WestVac BioPharma by taking advantage of the rapid response of the internationally advanced insect cell-based recombinant protein vaccine production technology platform and completing the construction of the vector in a highly efficient manner. The vaccine, targeting the receptor-binding domain (RBD) and heptad repeat (HR) domain of the spike protein of the XBB and other variants of COVID-19, develops subunit vaccine antigens that self-assemble into stable trimeric protein particles based on precise structure design. These particles are purified, mixed, and then combined with an oil-in-water emulsion adjuvant based on squalene. This innovative adjuvant significantly enhances the vaccine's neutralizing antibody titers and induces a stronger T-cell immune response in the body.
Clinical trial data showed that the Coviccine® Trivalent Vaccinecan induce high levels of neutralizing antibodies against XBB.1, XBB.1.5, XBB.1.16, XBB.1.9.1, EG.5, XBB.2.3, BA.5 and other variants, with good safety, and is of great significance for preventing variant infections, reducing severe cases, and lowering mortality rates.

It is reported that since May 2023, the World Health Organization (WHO), the U.S. Food and Drug Administration (FDA), the European Center for Disease Prevention and Control (ECDC) and the European Medicines Agency (EMA) have updated the ingredients of the 2023 COVID-19 vaccine in succession. It is recommended that the new vaccine should induce neutralizing antibody responses against the XBB variants that are currently circulating globally. WHO recommended the elderly, young people with severe comorbidities (such as diabetes and heart disease), immunocompromised people and front-line health workers should be given priority to receive the COVID-19 vaccine against the XBB variants.
In September 2023, the U.S. Centers for Disease Control and Prevention (CDC) officially made updated recommendations for the U.S. XBB mutated COVID-19 vaccine, recommending that everyone over 6 months old in the United States receive an updated COVID-19 vaccine with XBB.1.5 as the antigen in the fall and winter of 2023. According to U.S. government data, it showed that about 36 million adults have received the latest COVID-19 vaccine as of mid-November, which means that 13.9% of the adult population has gotten vaccinated with updated XBB COVID-19 vaccine in the two months since it was launched in the United States.
References:
1. National COVID-19 Infection Epidemic Situation. Chinese Center for Disease Control and Prevention website. 2023,9.https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202309/t20230906_269361.html
2.Callaway, E. COVID’s future: mini-waves rather than seasonal surges. Nature. 2023, 5. pp. 229‒230
3. Bowe, Benjamin, et al. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nature Medicine. 2022,11. pp. 2398-2405
4.WHO. SAGE updates COVID-19 vaccination guidance. SAGE updates COVID-19 vaccination guidance (who.int)
5.Statement on the antigen composition of COVID-19 vaccines.WHO. 2023,5. https:// www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines
6.EMA and ECDC statement on updating COVID-19 vaccines to target new SARS-CoV-2 virus variants.EMA.2023, 7. https:// www.ema.europa.eu/ en/news/ ema-ecdc- statement- updating-covid-19-vaccines-target-new-sars-cov-2-virus-variants
7. Package insert of Recombinant COVID-19 Trivalent (XBB + BA.5 + Delta) Protein Vaccine (Sf9 Cell)